In Europe, medical robots are regulated by the Medical Device Regulation (MDR), which ensures safety and quality but requires significant R&D investments. Despite the wide availability of industrial robots, these requirements limit their integration into medical robotic applications to operate near patients.
Identified unmet needs
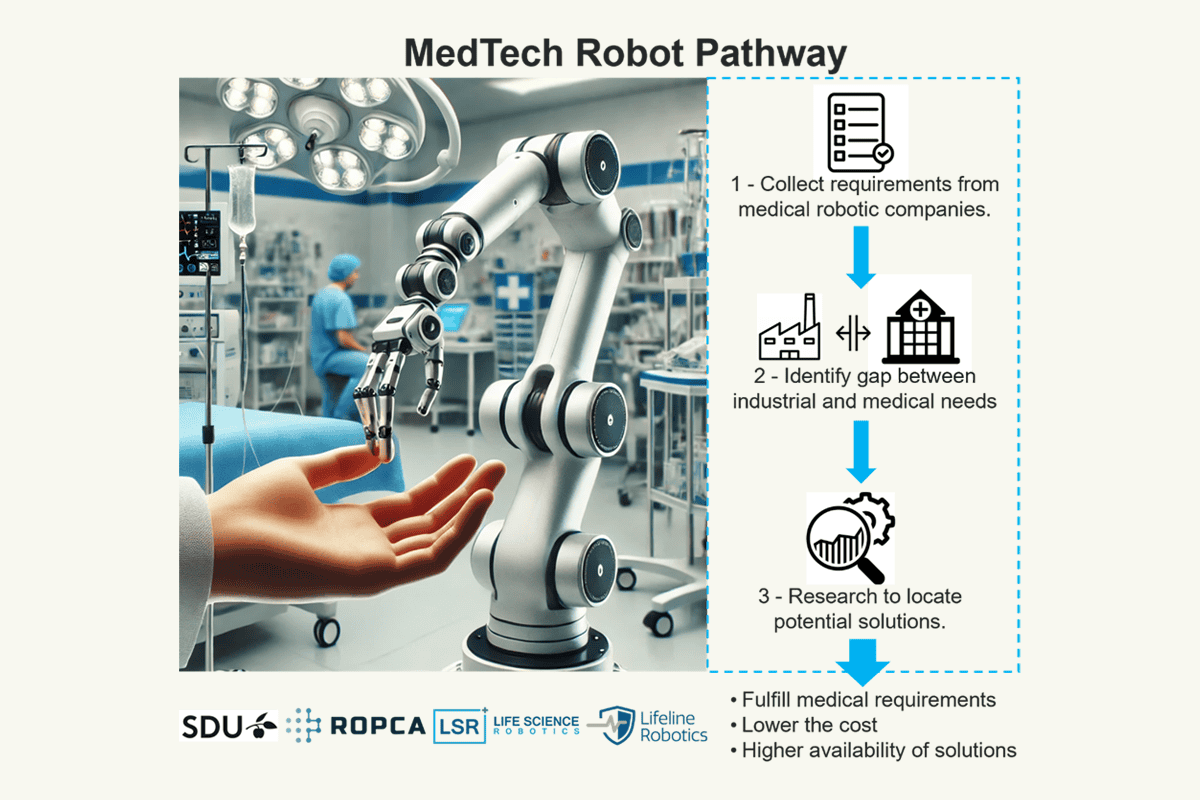
The MedTech Robot Pathway project identified unmet needs for medical robotic arms and explored alternatives to address the medical device industry’s demands. The findings showed that while industrial robots meet some medical requirements, they often lack important features for healthcare applications. Medical robots, unlike industrial ones, must prioritize other factors such as reduced weight, hidden connectors, and a design suitable for medical environments.
Common requirements:
Pose repeatability; Mounting flange; Installation position; Software; Power supply; Communication interfaces; Hardware interfaces; HRC features; Safety features.
Medical-specific requirements:
Total weight; Hidden connectors; Contact force estimation; Failure notification; Medical appearance; Risk management; Mean time to repair; Medical device standards compliance.
Task-dependent requirements:
Payload; Kinematic configuration; Force/torque accuracy; Maximum reach.
This gap highlights the need for more adaptable robotic designs for medical tasks. Customizing robotic arms to meet task-specific medical requirements could help reduce size and weight, offering a more cost-effective solution. However, this increased flexibility may introduce challenges, such as the need for certification and design modifications.
The lack of compliance
A major barrier remains the lack of compliance with medical device standards, particularly in electrical safety (IEC 60601-1), electromagnetic compatibility (EN 60601-1-2), and medical software (IEC 62304:2006). The high installation and maintenance costs of medical robots are also barriers to the medical robot’s market development.
As the medical robotics market grows, innovation is crucial. Solutions to address a broader range of task requirements while remaining cost-effective, adaptable, and compliant with the healthcare sector may sustain this growth.
The participants in the project:
- Syddansk Universitet – SDU
- ROPCA ApS
- Lifeline Robotics A/S
- Life Science Robotics ApS
For further information please contact:
Bruno Miguel Gomes Oliveira, SDU Robotics broli@mmmi.sdu.dk
The project has been funded by the Danish Agency for Higher Education and Science through Innovationskraftbevillingen 2021-2024.